Why doe Rf values of 02 and 01 separate better than Rf values of 03 and 02. Rf value is a ratio of the distance travelled on chromatography paper by a pigment considered the solute and the distance travelled by the solvent.

B 2 Calculating Retention Factor Rf Values Sl Youtube
What does the mobile phase describe.
. The outcome of these proceedings was that a person cannot absorb more than 16 watts of energy per kilogram of body weight. Notice that 1 the bigger the Rf the further the spot moved and 2 that the Rf should be the same for a component regardless of how far the solvent moves. The higher the R value is the better insulator it is.
Therefore the more different solvents or mixtures are used the more RF values are obtained and so the more concise the identification is. Since it is strongly adsorbed by silica gel it is difficult to move forward and the Rf value decreases. The higher the R Value the greater the resistance and better thermal insulating power.
They interact more with the silica gel thus travel less than non-polar compounds. More polar compound has higher Rf value than a nonpolar compound b more polar compound has the same Rf value as a nonpolar compound c more polar compound has lower Rf value than a nonpolar compound d. The rheumatoid factor RF blood test measures the level of the RF antibody in the blood.
For a linear regression. Rf Values for Identification Note that different componds can have the SAME Rf value for a particular solvent but unlikely to have similar Rf for a number 2-4 of different solvents. The best eluting solvent to use TLC analysis is the one that allows greater separation wider.
Which of the following is true. Furthermore a higher return loss value signifies less reflection in the wires. In chromatography a mixture of pigments to be measured is applied close to the bottom of a strip of chromatography.
RF 51 means fast encoding while very low quality. This upper limit of specific absorption rate is set well below the level of radiation that would endanger a users healthIt is the result of intense laboratory. Resulting in a lower Rf.
The R-squared value is the amount of variance explained by your model. What does the Rf value describe on a microscopic level. As a matter of fact the higher it is the better is your model.
Because a solute dissolves best into a solvent of like nature the higher the value of Rf the more that the solute is like the solvent. Homogeneity and normality of the data independence of the variables fixed X. Lower the RF value higher is the quality.
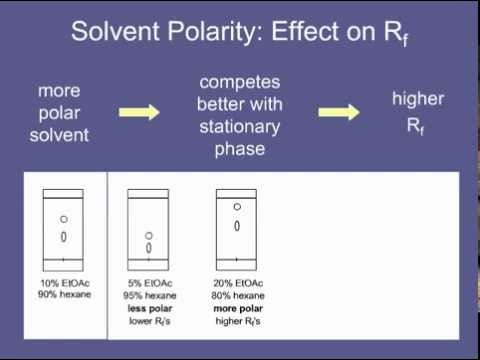
Which makes sense considering a polar compound will travel more in polar solvent and the polar solvent will compete for the stationary phase as well. Any compound that migrates in the solvent front has an Rf of 10. I hate it when people say stupid things.
So from this logic it would mean that if I want to encode a 360p video for YouTube I would have to select higher RF value because it is lower quality and conversely it I want a 1080p video then I must go for a lower RF value. Identical molecules means of two pigments has the same Rf value. Higher resolution with higher crf typically gives a better result.
Below 40-60 units per milliliter UMl. Retention factors are useful in comparing the results of one chromatogram to the results of another. RF 0 means very very slow but lossless.
There are two ways in which the normal rheumatoid factor range can be presented. Of course the Rf value is completely different depending on the developing solvent. Rf value Distance of the center of spot from the starting point or the distance travelled by the sample or analyte divided by Distance of solvent front from starting point or the distance travelled by the solvent front in chromatography.
I wish we would stop teaching chromatography in terms of polar and nonpolar The aspirin will interact fairly strongly with the silica due to hydrogen bondingelectrostatic interactions of the carboxylic acid and the ester with the silica. They created SAR as a mean of measuring how much RF your body can take when making a call. If the conditions in which the chromatogram are run are unchanged same mobile and stationary phases the retention.
Higher resolution with higher crf typically gives a better result. This means that a material with a high R value will require less thickness to insulate your home. It is impossible for any compound to migrate faster than the carrier solvent To maximize the discrimination or resolving power of paper chromatography or TLC the migration is best kept in the lower Rf ranges between 05 and 02.
Rf value or Retention factor is the difference in rate of movement of the components in chromatography is caused by various. See answer 1 Best Answer. Small Rf value means less soluble and pigments with Rf.
Simply put R Value is a term the construction industry uses to explain how well a material prevents heat from passing through a given material. It is a measure of how well your model fits your data. A better VSWR value results in a higher return loss and if the VSWR degrades then the return loss decreases as well.
A high Rf Ie 092 would refer to a substance that is very non-polar. On the other hand highly hydrophobic compounds are less likely to be adsorbed by silica gel so their Rf value is higher. The best separation occurs when the Rf values have the greatest multiplicative not additive differences.
If the molecule had a very high affinity for the stationary phase how would this affect the Rf value. Dont simply choose the cheapest insulating material possible because this wont guarantee to do the job properly. Rf value is an indicator of the solubility of a pigment.
OP CRF is a bit rate control method lower CRF values will produce in theory higher quality because they use more bit rate. For this reason we desire a loss in this. Why is this important.
The higher the ratio of ethyl acetate in the mobile phase the more. A low Rf value 010 would refer to a substance that is very polar. But is a higher or lower R Value better when it comes to energy efficiency.
Below 180 1 to 80 titer. What role does the mobile phase play in the distance a molecule travels in chromatography. However it only applies when te assumptions of the models are fulfilled eg.
Ie that substance moved a 92 of the entire distance the solvent traveled. In other words Rf distance moved by solute distance moved by solvent.

11 Solvent Polarity Effect On Rf Youtube
0 Comments